There is a rumor going around that the approval is for the name change. After looking at the Approval Letter from the FDA’s Comirnaty and Pfizer-BioNTech COVID-19 Vaccine page, it does appear to be the licensing letter for the inoculation. This is apparent in the terms used, which are different from those in an Emergency Use Authorization. Compare the language used in the approval letter with those issued with the EUAs. It looks like there are still some final details that are being worked out; otherwise, it appears that the Comirnaty inoculation is now fully approved for sale. As of yet, the Comirnaty jab is not available in the US. The Comirnaty/Pfizer-BioNTech Fact Sheet for Healthcare Providers Administering Vaccine clearly states that pre-authorization Pfizer-BioNTech vaccine and the Comirnaty vaccine are equivalent. However, a recent judicial ruling claims that there is no legal equivalency, and they are not interchangeable.
- October 20, 2021. “Fact Sheet for Healthcare Providers Administering Vaccine [Pfizer-BioNTech].” US Food & Drug Administration. https://www.fda.gov/media/144413/download.

Food & Drug Administration.
This document has instructions for healthcare providers who are administering the vaccines.
However, another letter from August 23, 2021, admits that there are differences between the Pfizer-BioNTech vaccine and the Comirnaty vaccine, though they are not spelled out.
The licensed vaccine has the same formulation as the EUA-authorized vaccine and the products can be used interchangeably to provide the vaccination series without presenting any safety or effectiveness concerns. The products are legally distinct with certain differences that do not impact safety or effectiveness.
The FDA is saying that the products are legally distinct. They are not the same. A case can be made that if the recipient did not receive a jab that did came out of a vial with a Comirnaty label, they did not receive the approved vaccine.
- August 23, 2021. Denise M. Hinton. “FDA Letter to Pfizer.” US Food & Drug Administration.

https://web.archive.org/web/20210823142034/https://www.fda.gov/media/150386/download.
Food & Drug Administration.
The FDA has used this same URL to refer to various versions of this letter over time. This is the first version of the letter saying that Comirnaty and Pfizer-BioNTech are legally distinct.
Local copy. - August 24, 2021. Patrick Wood. “FDA/Media Shell Game: Pfizer ‘Vaccine’ Was Not Approved After All.” Technocracy News & Trends.

https://www.technocracy.news/fda-media-shell-game-pfizer-vaccine-not-approved-after-all/.
News.
The Approval Letter ends thus:
POST APPROVAL FEEDBACK MEETING
New biological products qualify for a post approval feedback meeting. Such meetings are used to discuss the quality of the application and to evaluate the communication process during drug development and marketing application review. The purpose is to learn from successful aspects of the review process and to identify areas that could benefit from improvement. If you would like to have such a meeting with us, please contact the Regulatory Project Manager for this application.
So, if we have concerns, we have been invited to share them.
Meanwhile, the trials have NOT been completed. Furthermore, the trial has already been unblinded and is thus no longer a randomized control trial. The Phase III trial is not scheduled for completion until May 2, 2023. In other words, Comirnaty was approved prior to completion of required trials.
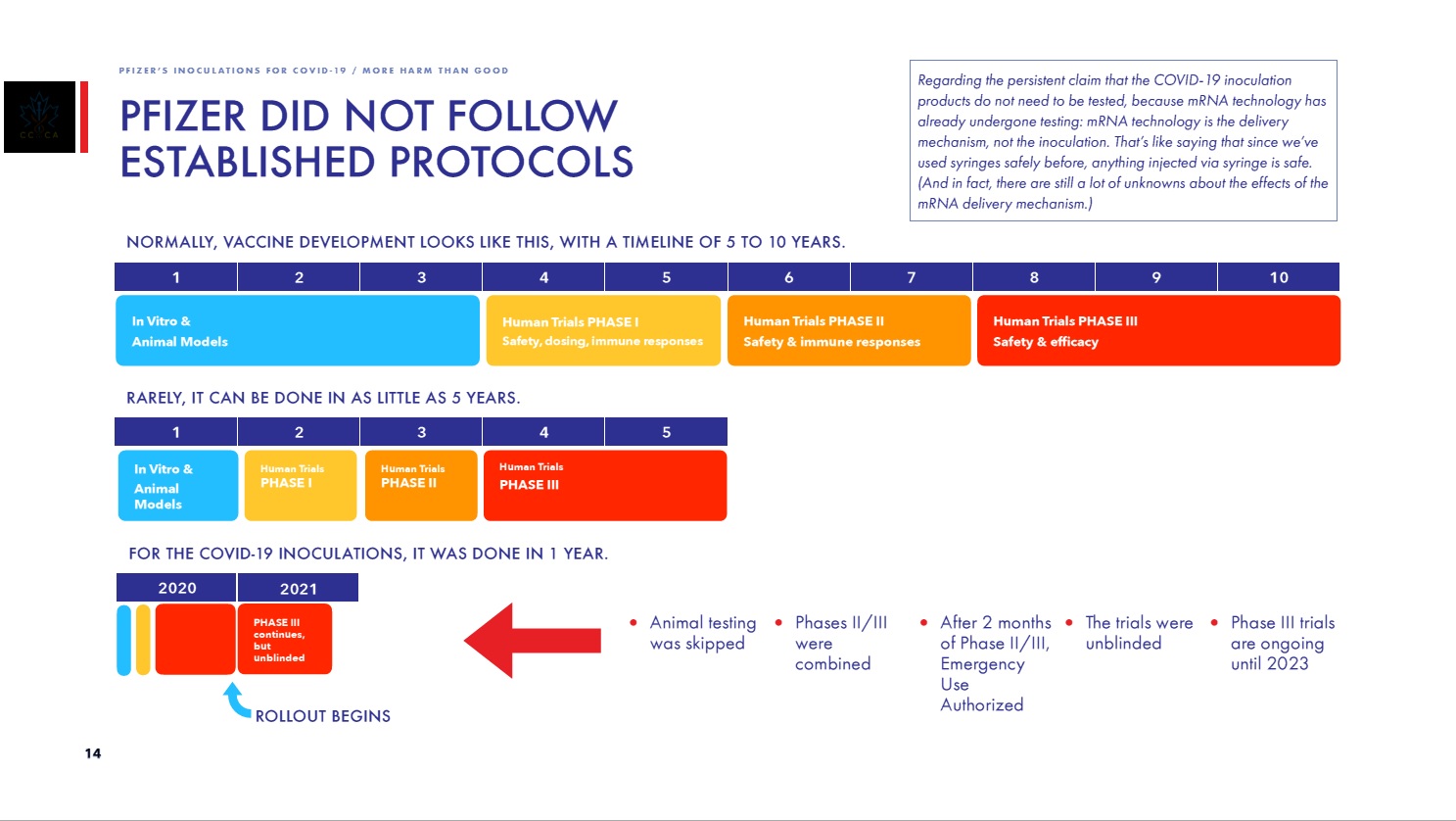
Page 14 of CCCA report: Pfizer did not follow established protocols
I believe that Dr. Jane Ruby in the interview below has properly assessed the situation. In discussing the proposed Comirnaty package insert, she said:
This document is so paltry, I have to tell you, in my estimation, the FDA itself has violated its own federal [regulations]. In that situation, to me, this is an illegal approval, and the FDA is committing crimes.
— Dr. Jane Ruby
Compare the proposed Comirnaty package insert to inserts for other products, and the differences in quality of content become obvious.
Dr. Ruby then goes on to list some 20 alleged crimes that were committed just in the package insert.
Dr. Ruby offers a ray of hope: 50% of those inoculated received a placebo.
The ingredients list is due on September 7.
Sources:
- September 3, 2021. “Pfizer-BioNTech/COMIRNATY Vaccine Is Still Under ‘STUDY’ Runs to Be Completed at Different Intervals Between 2022-2026.” Expanding Awareness Relations.

https://www.expandingawarenessrelations.com/pfizer-biontech-comirnaty-vaccine-is-still-under-study-runs-to-be-completed-at-different-intervals-between-2022-2026/.
Blog.
This person did an excellent job of analyzing the documents. It will still be a long time before all safety studies are completed. Highly recommended. - August 23, 2021. “FDA Approves First COVID-19 Vaccine.” US Food & Drug Administration.

https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine.
Food & Drug Administration. - August 23, 2021. “Comirnaty and Pfizer-BioNTech COVID-19 Vaccine Frequently Asked Questions.” US Food & Drug Administration.

https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/comirnaty-and-pfizer-biontech-covid-19-vaccine-frequently-asked-questions.
Food & Drug Administration. - “Comirnaty and Pfizer-BioNTech COVID-19 Vaccine.” US Food & Drug Administration.

https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine.
Food & Drug Administration.
This page has links to official communications with Pfizer. - August 2021. “Comirnaty Package Insert.” US Food & Drug Administration.

https://www.fda.gov/media/151707/download.
Food & Drug Administration. - August 24, 2021. Center for Biologics Evaluation and Research. “Comirnaty.” US Food & Drug Administration.

https://www.fda.gov/vaccines-blood-biologics/comirnaty.
Food & Drug Administration.
Comirnaty has its own page now as well. - August 23, 2021. Mary A. Malarkey and Marion F. Gruber. “August 23, 2021 Approval Letter.” US Food & Drug Administration.

https://www.fda.gov/media/151710/download.
Food & Drug Administration. - August 23, 2021. Bruce Y. Lee. “Why ‘Comirnaty’ Is The New Name For Pfizer Covid-19 Vaccines, ‘Spikevax’ For Moderna.” Forbes.

https://www.forbes.com/sites/brucelee/2021/08/23/why-comirnaty-is-the-new-name-for-pfizer-covid-19-vaccines-spikevax-for-moderna/.
Magazine. - August 23, 2021. Stew Peters with Jane Ruby. “FDA Approval ILLEGAL! Doctor Reveals Pfizer Insert Proves Criminal Regulation Violations!” The Stew Peters Show, Red Voice Media. Runtime of embedded video: 19:23.


https://www.redvoicemedia.com/video/2021/08/fda-approval-illegal-doctor-reveals-pfizer-insert-proves-criminal-regulation-violations/.
News, Video. - August 25, 2021. Stew Peters with Karen Kingston. “Former Pfizer Employee: ‘Checkmate. Game Over. We WIN.’” Red Voice Media, The Stew Peters Show. Runtime of embedded video: 32:19.


https://www.redvoicemedia.com/2021/08/former-pfizer-employee-checkmate-game-over-we-win/.
News, Video.
Links to documents mentioned in the video are in the description text on this page. - August 26, 2021. T. L. B. Staff. “Pfizer Has to Come Clean on Ingredients & Harms Within 14 Days.” Europe Reloaded. Runtime of embedded video: 32:19 [same video as above].


https://www.europereloaded.com/pfizer-has-to-come-clean-on-ingredients-harms-within-14-days/.
News, Video.
This article is notes from the Stew Peters interview with Karen Kingston listed just above. - August 26, 2021. Nicholas Veniamin with Alan Fountain. Alan Fountain Discusses FDA, Pfizer, Sting Operation with Nicholas Veniamin. Nicholas Veniamin.

https://www.bitchute.com/video/MsYRnuUDs7oF/.
Video. - March 24, 2023. Jikkyleaks 🐭 [@Jikkyleaks]. “@NarfGb @DoorlessCarp [KNOWINGLY] Remember Why They Called It Co-MiRNA-Ty? Https://Pubmed.Ncbi.Nlm.Nih.Gov/25515214/ Https://T.Co/Jtfi1ErlRK.” Tweet. Twitter.

https://twitter.com/Jikkyleaks/status/1639149379838943232.
Social Media. - “Comirnaty Pronunciation.”


https://www.howtopronounce.com/comirnaty.
Reference, Audio.
Related:
- July 1, 2021. Piero Olliaro, Els Torreele, and Michel Vaillant. “COVID-19 Vaccine Efficacy and Effectiveness—the Elephant (Not) in the Room.” The Lancet Microbe 2 (7): e279–80.

https://doi.org/10.1016/S2666-5247(21)00069-0.
Research Journal. - June 1, 2021. Piero Olliaro. “What Does 95% COVID-19 Vaccine Efficacy Really Mean?” The Lancet Infectious Diseases 21 (6): 769.

https://doi.org/10.1016/S1473-3099(21)00075-X.
Research Journal. - December 16, 2021. The Pfizer Inoculations Do More Harm Than Good. CanadianCovidCareAlliance.

https://rumble.com/vqx3kb-the-pfizer-inoculations-do-more-harm-than-good.html.
Video. - December 16, 2021. “The Pfizer Inoculations for COVID-19: More Harm Than Good.” Canadian Covid Care Alliance.



https://www.canadiancovidcarealliance.org/wp-content/uploads/2021/12/The-COVID-19-Inoculations-More-Harm-Than-Good-REV-Dec-16-2021.pdf.
Doctor Organization, Activist, PDF.
#CCCA #MoreHarmThanGood - April 5, 2022. FDA APPROVALGATE: Fake Approval Scandal, Only EUA Is Available… All by Design! Tim Truth.

https://www.bitchute.com/video/dXKMnh7ZQQ3o/.
Video. - June 11, 2022. Zach Heilman. “CDC Publishes Hidden Pfizer Document With An Admission That Proves Military Jab Mandates Illegal [VIDEO].” Red Voice Media. Runtime of embedded video: 2:49.

https://www.redvoicemedia.com/2022/06/cdc-publishes-hidden-pfizer-document-with-an-admission-that-proves-military-jab-mandates-illegal-video/.
News.
They had to ignore a whole lot to approve the inoculation. This is not about health.