
The study presented in the Emergency Use Authorization based its efficacy assessment on the results of PCR tests, which we now know are unreliable and prone to errors. Page 41 includes additional data about people who actually got sick after the inoculation. Significantly more people got sick in the test group after vaccination than in the control group. This information was charted by Karen Kingston and is presented toward the end of this post.
Under section 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), when the Secretary of HHS declares that an emergency use authorization is appropriate, FDA may authorize unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by CBRN threat agents when certain criteria are met, including there are no adequate, approved, and available alternatives. — FDA statement on EAUs
In other words, if there is a viable option, an EAU should not be granted.

From page 4 of the Letter of Authorization, August 12, 2021
Effective treatment protocols using approved drugs existed prior to the first issuance of an EUA for vaccines. Doctors testified of this in the Senate.
Safety and efficacy testing is still ongoing for this product. Details are in the Review Memorandum found in the FDA’s main page for this product. Therefore, no advertising for this product may legally use the terms “safe and effective”.
Pfizer is required to report to VAERS any adverse reaction, whether or not is is attributed to the vaccine.

From page 8 of the Letter of Authorization, August 12, 2021
Was this reporting to VAERS done as required?
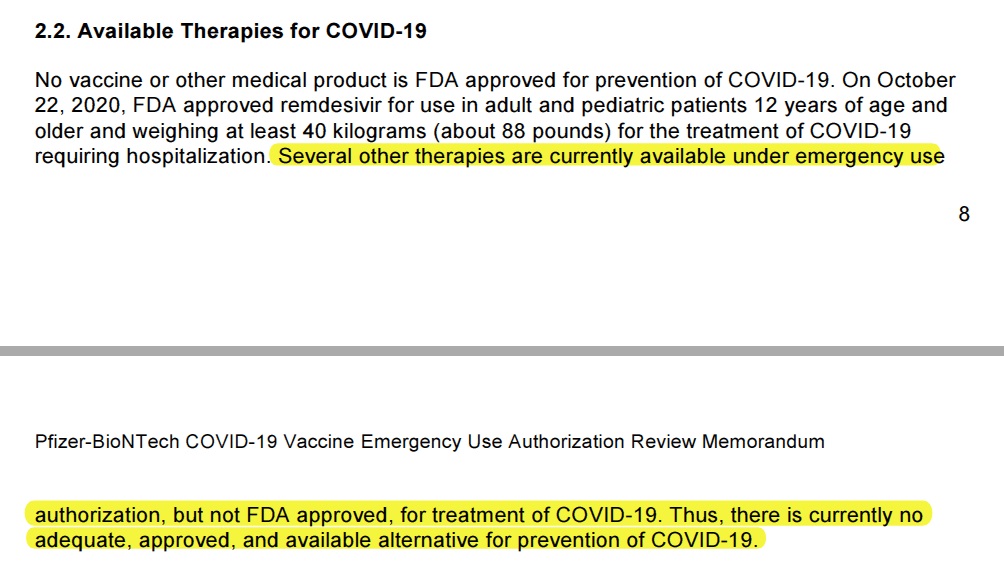
Statement about available therapies
The EUA acknowledges in the clip above that other therapies are available, but they have not been FDA approved specifically for the treatment of COVID-19. In saying this, they are admitting that they are relying on a technicality to qualify the vaccine for the EUA. An EUA should only be issued if there are no other options. Other options existed, but they had not been specifically approved for COVID-19 and thus were disregarded.
How long would it have taken for the other options to have been relabeled as treatment for COVID-19? Was it possible in the timeframe available up until this point? Should these other options have been disregarded in this way, given the clinical results being reported by those using these other options? Was there intent by the FDA to disregard? What was the motivation?
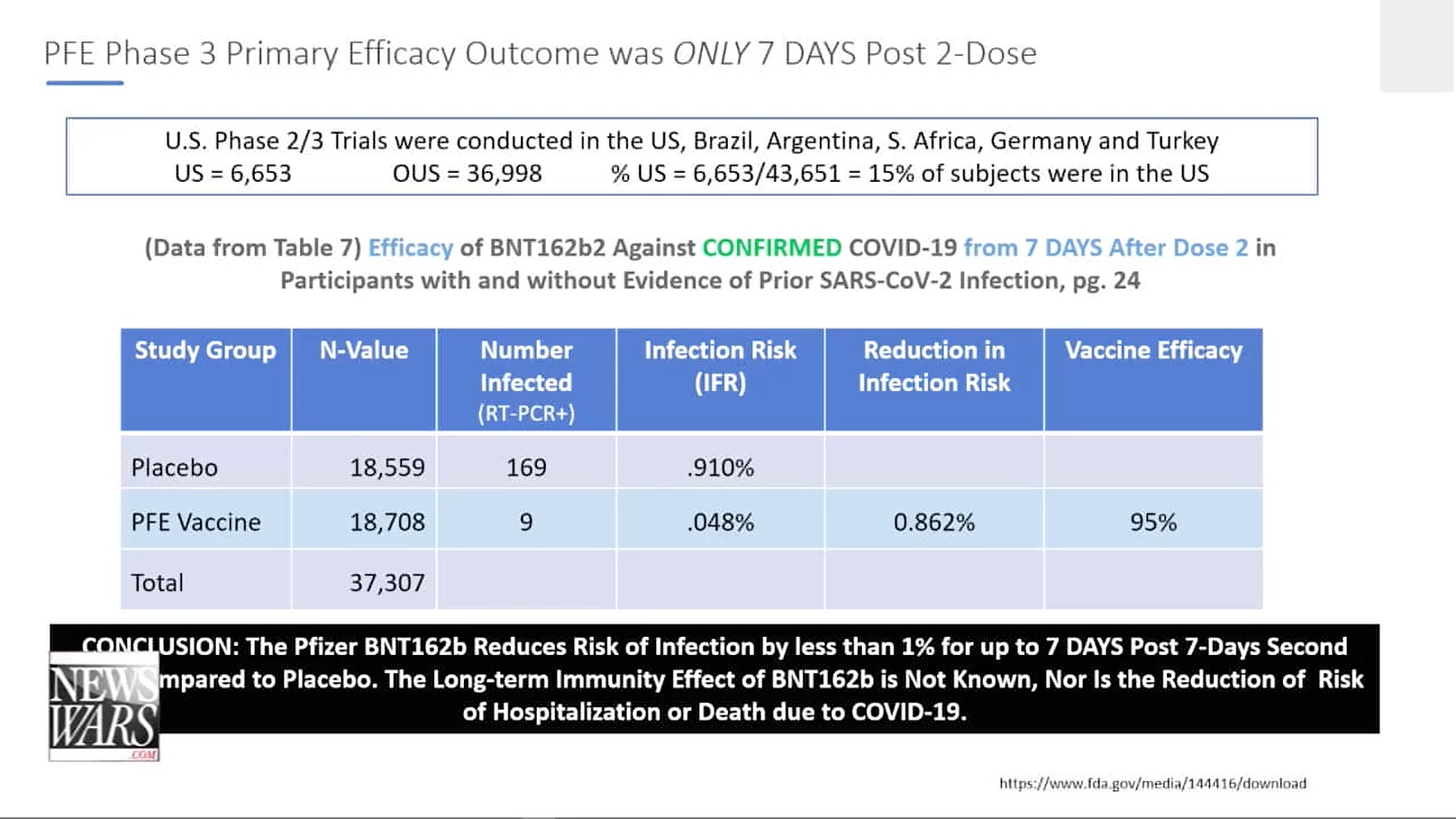
Charted by Karen Kingston: This is the efficacy that is being claimed.
Note that the follow-up period in the chart above is only 7 days. Karen Kingston found additional data in the report beyond 7 days, and the following chart shows those results. Also note that they were using PCR tests to determine who had been infected without regard for whether the person had symptoms.
How many cycles were used in evaluating the PCR tests? Were the same number of cycles used for both groups?
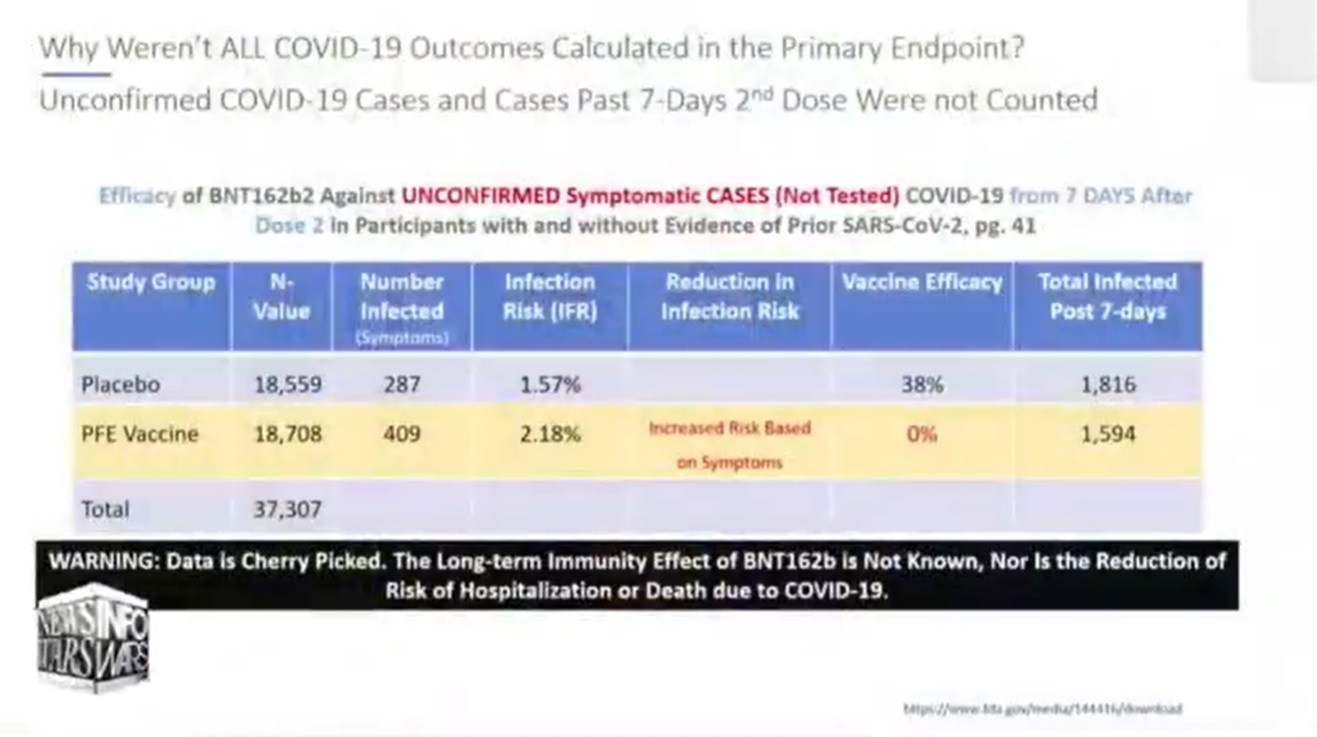
Charted by Karen Kingston: These are the results when all data is charted.

Text on page 41 from which chart of unconfirmed illness was derived
The above chart shows how many got sick as evidenced by symptoms. More unvaccinated people in the study may have tested positive on a PCR test, but this data shows that more vaccinated people actually had symptoms of being sick. And isn’t that really what matters?
I’m going to go out on a limb here. I am not a medical expert.
If more unvaccinated test subjects tested positive on a PCR test, but more vaccinated test subjects had symptoms, might this be early evidence of the ADE dangers of the vaccines predicted by industry experts?
Sources:
- US Food & Drug Administration. “Pfizer-BioNTech COVID-19 Vaccine.”

https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine.
Food & Drug Administration.
This is the FDA’s main page for the Pfizer-BioNTech COVID-19 Vaccine. Links to FDA letters, memos, reports, and other documents relating the Pfizer-BioNTech COVID-19 Vaccine can be found here. - November 20, 2020. US Food & Drug Administration. “Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum.” [Pfizer-BioNTech]

https://www.fda.gov/media/144416/download.
Food & Drug Administration. - US Food & Drug Administration. “Emergency Use Authorization.”

https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization.
Food & Drug Administration.
This is an information page describing the Emergency Use Authorization policies. - August 4, 2021. Andrew Kaufman with Karen Kingston. BOMBSHELL: Pfizer Whistleblower Confirms Covid Injections Are Poisonous Bioweapons. The Alex Jones Show.


https://freeworldnews.tv/watch?id=610b15ec4b401c2c26621b15.
News, Video.
Karen Kingston’s chart is a screen print from this video.
See also, on this site:
- August 22, 2005: Virology Journal: “Chloroquine is a potent inhibitor of SARS coronavirus infection and spread”
- July 2, 2020: Dr. Vladimir Zelenko publishes his COVID-19 treatment protocol.
- November 19, 2020: The United States Senate holds a hearing on Senate Hearing on COVID-19 Outpatient Treatment.