
Grant Final Report: Review of Vaccine Adverse Event Reporting System (VAERS) determines that fewer than 1% of vaccine adverse events are reported. Even if we assume that the COVID-19 situation has created increased awareness of VAERS, we can assume that adverse reactions are still grossly underreported. If nothing has changed, this means that statistics derived from VAERS can be multiplied by 100.
In addition to being grossly underreported, it appears that the data in VAERS may not be entirely up to date. It appears that the CDC is behind in their reporting of adverse reactions.
According to Dr. David Martin, the National Childhood Vaccine Injury Act of 1986 requires that the VAERS database be maintained accurately. This is a statutory requirement for maintaining immunity.
It is important to note that VAERS has a very significant reporting backlog. Over 150,000 adverse events have been reported, but not yet included in the publicly available database. In many cases, there is a three month delay.
— Good King Elliot (@GoodKingElliot) June 23, 2021
The following shows how VAERS data compares with the Pfizer clinical trial data.
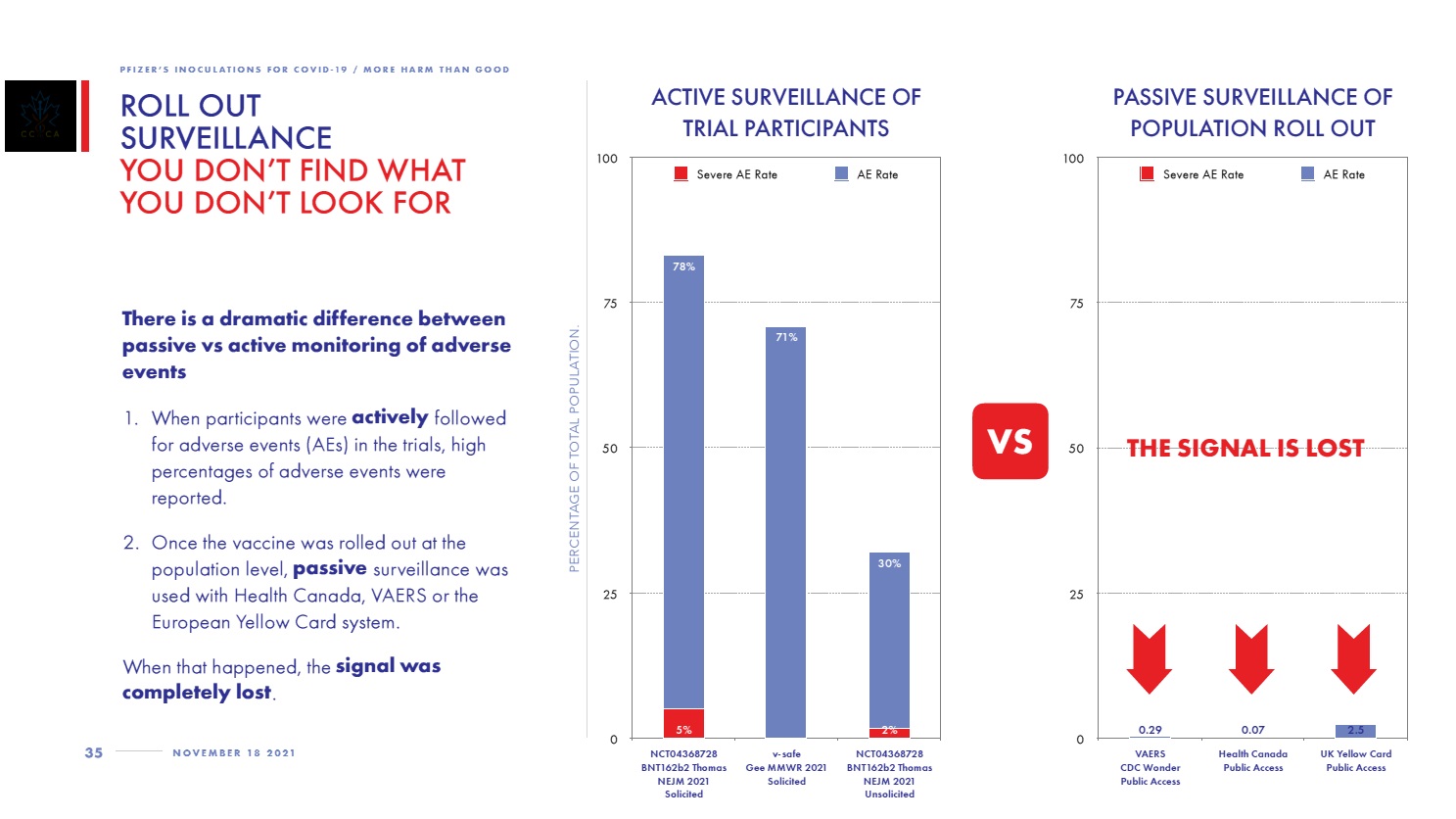
Page 35 of CCCA report: Roll Out Surveillance: You Don’t Find What You Don’t Look For
Left side chart is the Pfizer clinical trials. Right side chart is the passive surveillance of the general population post-rollout.
Sources:
- September 30, 2010. Ross Lazarus, Michael Klompas, and Steve Bernstein. “Electronic Support for Public Health–Vaccine Adverse Event Reporting System (ESP:VAERS).” Grant Final Report, Grant ID: R18 HS 017045. The Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services.


https://digital.ahrq.gov/sites/default/files/docs/publication/r18hs017045-lazarus-final-report-2011.pdf.
Government, PDF. - Centers for Disease Control and Prevention. “The Vaccine Adverse Event Reporting System (VAERS).”

https://wonder.cdc.gov/vaers.html.
Centers for Disease Control and Prevention. - U.S. Department of Health and Human Services (HHS). “Vaccine Adverse Event Reporting System (VAERS).”

https://vaers.hhs.gov/.
Government. - “VAERS COVID Vaccine Data.” OpenVAERS.

https://www.openvaers.com/covid-data.
Statistics. - June 23, 2021. Good King Elliot. “@RWMaloneMD It Is Important to Note That VAERS Has a Very Significant Reporting Backlog. Over 150,000 Adverse Events Have Been Reported, but Not yet Included in the Publicly Available Database. In Many Cases, There Is a Three Month Delay.” Tweet. @GoodKingElliot (blog).

https://twitter.com/GoodKingElliot/status/1407727432166223872.
Social Media. - December 23, 2021. Seth Holehouse with David Martin. Why Does Trump Keep Promoting the Vaccine? Man in America. Runtime: 1:06:35.


https://rumble.com/vrahql-why-is-trump-promoting-the-vaccine.html.
Video, Expert.
Related:
- U.S. Health Resources & Services Administration. “Countermeasures Injury Compensation Program (CICP).”

https://www.hrsa.gov/cicp.
Government. - August 26, 2015. Tom T. Shimabukuro, Michael Nguyen, David Martin, and Frank DeStefano. “Safety Monitoring in the Vaccine Adverse Event Reporting System (VAERS).” Vaccine 33 (36): 4398–4405.

https://doi.org/10.1016/j.vaccine.2015.07.035.
Research Journal. - December 16, 2021. The Pfizer Inoculations Do More Harm Than Good. CanadianCovidCareAlliance.

https://rumble.com/vqx3kb-the-pfizer-inoculations-do-more-harm-than-good.html.
Video. - December 16, 2021. “The Pfizer Inoculations for COVID-19: More Harm Than Good.” Canadian Covid Care Alliance.



https://www.canadiancovidcarealliance.org/wp-content/uploads/2021/12/The-COVID-19-Inoculations-More-Harm-Than-Good-REV-Dec-16-2021.pdf.
Doctor Organization, Activist, PDF.
#CCCA #MoreHarmThanGood
See also, on this site: