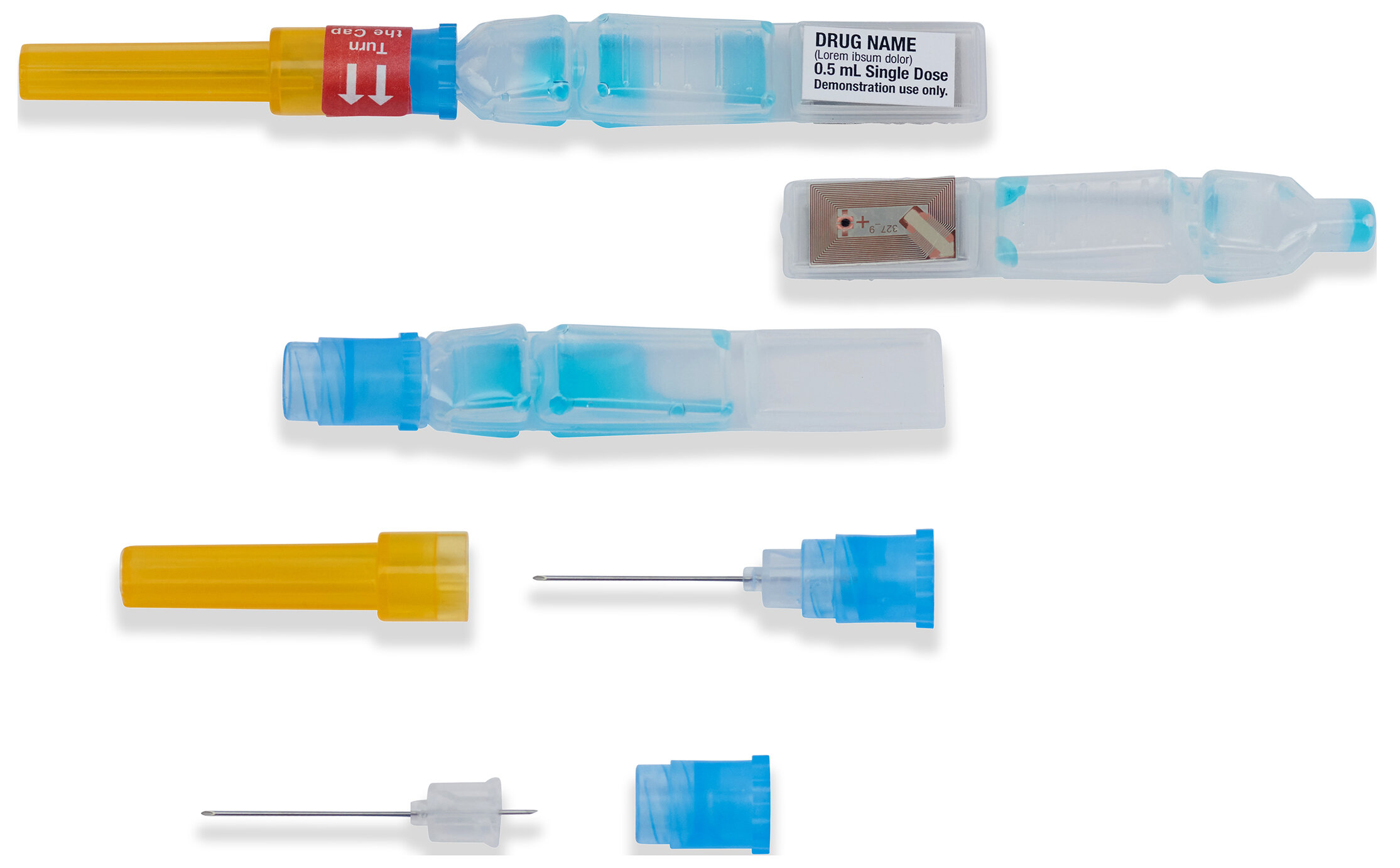

near-field communication (NFC) tag
Available information shows a RFID (NFC) tag that is used to track the syringe itself. ApiJect has used both terms. The tag is an IoT (Internet of Things) device.
At the time of this writing in December 2021, it does not appear that the ApiJect products are being used. The jabs are being delivered to administrators in multi-dose vials. The ApiJect product is for single dose usage. I do not know if any kind of special syringes are being used for jab administration or who the manufacturer would be.
Sources:
- May 12, 2020. Mike Andrews. “DOD Awards $138 Million Contract Enabling Prefilled Syringes for Future COVID-19 Vaccine.” U.S. Department of Defense.

https://www.defense.gov/News/Releases/Release/Article/2184808/dod-awards-138-million-contract-enabling-prefilled-syringes-for-future-covid-19/.
Department of Defense. - “Welcome to ApiJect.” ApiJect.

https://apiject.com/.
Business. - Rapid Consortium.

https://rapidconsortium.org/.
Business.
RAPID USA, Inc. is a subsidiary of ApiJect Systems America, Inc. - “When COVID-19 Vaccine and Injectable Therapeutics Become Available, the RAPID Consortium Can Ease a U.S. Supply Chain Problem by Filling and Finishing 300+ Million Prefilled Syringes per Month.” 70207_37. The RAPID Consortium.


https://apiject.com/wp-content/uploads/2020/08/70207_apiject-bluebklt_184-min.pdf.
Business, PDF.
This document describes how the RFID (NFC) tag is used, beginning on print page 74, PDF page 41. - May 13, 2020. Richard Hunt and U.S. Department of Defense. “RFID Chip Tech ‘Could’ Be Used: Dept. Of Defense & HHS Award Contract For 100-M Prefilled Syringes For Future COVID-19 Vaccine.” Positive Encouraging K-LOVE.

https://www.klove.com/news/health/rfid-chip-tech-could-be-used-dept-of-defense-and-hhs-award-contract-for-100-m-prefilled-syringes-for-future-covid-19-vaccine-12564.
News.
Related:
- March 14, 2012. Nathan Chandler. “What’s an NFC Tag?” HowStuffWorks.

https://electronics.howstuffworks.com/nfc-tag.htm.
Reference.
See also, on this site:
Whether there is an injectable chip inside the contracted product, as some reports have suggested, is not known to me at this time. Given the reports of Bluetooth connectivity, I cannot rule out the possibility; but I cannot confirm it, either. Please see the PDF brochure for published information about how the NFC tag is intended to be used.